SAN DIEGO – August 14, 2024 (Investorideas.com Newswire) Aethlon Medical, Inc. (Nasdaq: AEMD), a medical therapeutic firm centered on growing merchandise to deal with most cancers and life-threatening infectious ailments, at this time reported monetary outcomes for its fiscal first quarter ended June 30, 2024 and supplied an replace on latest developments.
Paid Information Dissemination of behalf of AEMD.
Firm Updates
Aethlon Medical is continuous the analysis and scientific growth of its Hemopurifier®, a therapeutic blood filtration system designed to bind and take away dangerous exosomes and life-threatening viruses from blood and different organic fluids. These qualities of the Hemopurifier have potential functions in oncology, the place most cancers related exosomes might promote immune suppression and metastasis, in life-threatening infectious ailments, and in organ transplantation.
As introduced on August 12, 2024, the Bellberry Human Analysis Ethics Committee (BHREC) granted full ethics approval to the Pindara Personal Hospital for a security, feasibility and dose-finding scientific trial of the Hemopurifier® in sufferers with strong tumors who’ve steady or progressive illness throughout anti-PD-1 monotherapy therapy, reminiscent of Merck’s Keytruda® (pembrolizumab) or Bristol Myers Squibb’s Opdivo® (nivolumab) (AEMD-2022-06 Hemopurifier Examine). The approval is legitimate for one 12 months, till August 6, 2025. The trial will likely be performed by Dr. Marco Matos and his workers on the Pindara Personal Hospital, positioned in Queensland, Australia.
Earlier, on June 18, 2024, the Human Analysis Ethics Committee (HREC) of the Central Adelaide Native Well being Community (CALHN) granted full ethics approval for a similar security, feasibility and dose-finding scientific trial of the Hemopurifier in most cancers sufferers with strong tumors who’ve steady or progressive illness throughout anti-PD-1 monotherapy therapy, reminiscent of Keytruda® (pembrolizumab) or Opdivo® (nivolumab) (AEMD-2022-06 Hemopurifier Examine). The approval is legitimate for 3 years, till June 13, 2027. The trial will likely be performed by Prof. Michael Brown and his workers on the Most cancers Medical Trials Unit, CALHN, Royal Adelaide Hospital, positioned in Adelaide, Australia.
At the moment, solely roughly 30% of sufferers who obtain pembrolizumab or nivolumab could have lasting scientific responses to those brokers. Extracellular vesicles (EVs) produced by tumors have been implicated within the unfold of cancers in addition to the resistance to anti-PD-1 therapies. The Aethlon Hemopurifier has been designed to bind and take away these EVs from the bloodstream, which can enhance therapeutic response charges to anti-PD-1 antibodies. In preclinical research, the Hemopurifier has been proven to scale back the variety of EVs in most cancers affected person plasma samples.
“Throughout the fiscal first quarter and subsequent interval, now we have continued to make important progress advancing in the direction of our deliberate oncology trials in Australia and India, punctuated by the latest approval from the Bellberry Human Analysis Ethics Committee (BHREC), which granted full ethics approval to the Pindara Personal Hospital and earlier from the Human Analysis Ethics Committee at Central Adelaide Native Well being Community, in June, for a security, feasibility and dose-finding scientific trial of the Hemopurifier in sufferers with strong tumors who’ve steady or progressive illness throughout anti-PD-1 monotherapy therapy,” acknowledged James Frakes, Interim Chief Govt Officer and Chief Monetary Officer of Aethlon Medical.”
“Going ahead, the subsequent steps are to obtain approval from the Analysis Governance Workplace at every hospital, which critiques indemnities and insurance coverage. As soon as these approvals are obtained, Aethlon, in live performance with our Australian Contract Analysis Group, ReSQ, will conduct Web site Initiation Visits (SIVs), after which affected person enrollment within the trials might proceed. We count on that we are going to be open for enrollment in October 2024.”
Mr. Frakes continued, “We anticipate a number of upcoming, potential value-creating milestones, together with submission to the Ethics Committees at a 3rd website in Australia and one in website in India, with the expectation of probably receiving approval from one or each of these hospitals within the September or December quarter of 2024. After approval is granted, we count on to have the ability to enroll sufferers at these further websites by the top of 2024.”
As a reminder, the first endpoint of the approximate 9 to 18-patient, security, feasibility and dose-finding trial, is security. The trial will monitor any antagonistic occasions and clinically important adjustments in lab exams of Hemopurifier handled sufferers with strong tumors with steady or progressive illness at totally different therapy intervals, after a two-month run in interval of PD-1 antibody, Keytruda® or Opdivo® monotherapy. Sufferers who don’t reply to the PD-1 antibody remedy will likely be eligible to enter the Hemopurifier interval of the research the place sequential cohorts will obtain 1, 2 or 3 Hemopurifier therapies throughout a one-week interval. Along with monitoring security, the research is designed to look at the variety of Hemopurifier therapies wanted to lower the focus of EVs and if these adjustments in EV concentrations enhance the physique’s personal pure capability to assault tumor cells. These exploratory central laboratory analyses are anticipated to tell the design of subsequent efficacy and security trials, together with a Premarket Approval (PMA) research required by the FDA and different regulatory companies.
The corporate continues to discover alternatives to develop using the Hemopurifier as a therapy for life-threatening viral infections. In vitro, it has proven effectiveness in capturing viruses reminiscent of Zika, Lassa, MERS-CoV, cytomegalovirus, Epstein-Barr, Herpes simplex, Chikungunya, Dengue, West Nile, smallpox-related viruses, H1N1 swine flu, H5N1 hen flu, Monkeypox, and the reconstructed 1918 Spanish flu virus. The corporate’s COVID-19 trial in India stays open to accommodate any potential COVID-19 admissions to the intensive care models on the two taking part websites, Medanta Medicity Hospital and Maulana Azad Medical School. To this point, one affected person has been handled. The corporate is actively evaluating COVID-19 admissions and potential enrollment towards the continuing prices of sustaining the trial.
Monetary Outcomes for the Fiscal First Quarter Ended June 30, 2024
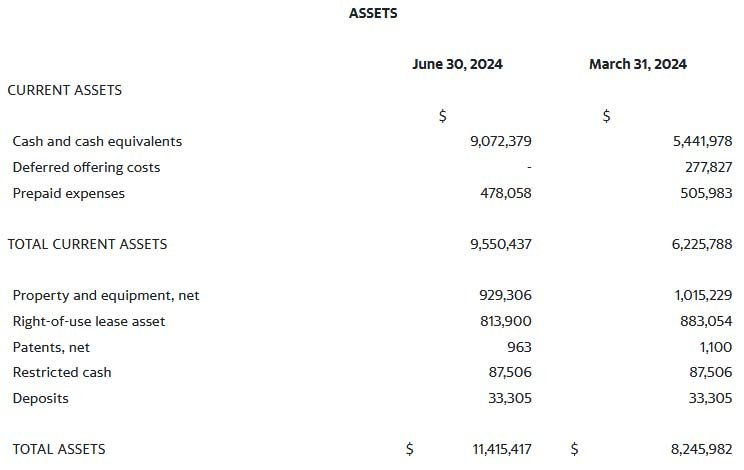
As of June 30, 2024, Aethlon Medical had a money stability of roughly $9.1 million.
Consolidated working bills for the fiscal quarter ended June 30, 2024 had been roughly $2.6 million in comparison with $3.4 million for the fiscal quarter ended June 30, 2023. This lower of roughly $800,000, or roughly 24%, within the 2024 interval was attributable to a lower of roughly $600,000 normally and administrative bills and a lower of roughly $300,000 in skilled charges partially offset by a rise in payroll and associated bills of roughly $100,000.
The $600,000 lower normally and administrative bills within the fiscal quarter ended June 30, 2024 was primarily attributable to a $447,000 lower in provides associated to the acquisition of uncooked supplies for manufacturing of the Hemopurifier and for lab provides.
The approximate $300,000 lower in skilled charges was primarily attributable to a $136,000 lower in consulting bills primarily associated to termination of companies with a contract manufacturing group, a $110,000 lower in scientific consulting, and a $78,000 lower in authorized charges referring to basic company issues.
The approximate $100,000 enhance in payroll and associated was primarily attributable to a rise in separation bills of roughly $300,000 associated to the termination of an worker. That enhance was offset by a lower of $111,000 in stock-based compensation and an $89,000 lower normally and administration personnel expense.
Because of the elements famous above, the corporate’s web loss decreased to roughly $2.6 million within the fiscal quarter ended June 30, 2024 from roughly $3.3 million within the fiscal quarter ended June 30, 2023.
The consolidated stability sheet for June 30, 2024, and the consolidated statements of operations for the fiscal quarters ended June 30, 2024 and 2023 comply with on the finish of this launch.
Convention Name
Administration will host a convention name at this time, Wednesday, August 14, 2024, at 4:30 p.m. ET to overview the corporate’s monetary outcomes and up to date company developments. Following administration’s formal remarks, there will likely be a query and reply session.
events can register for the convention by navigating to https://dpregister.com/sreg/10191735/fd44630e3d . Please be aware that registered members will obtain their dial-in quantity upon registration.
events with out web entry or unable to pre-register might dial in by calling:
PARTICIPANT DIAL IN (TOLL FREE): 1-844-836-8741
PARTICIPANT INTERNATIONAL DIAL IN: 1-412-317-5442
All callers ought to ask for the Aethlon Medical, Inc. convention name.
A replay of the decision will likely be obtainable roughly one hour after the top of the decision by September 14, 2024. The replay will be accessed by way of Aethlon Medical’s web site or by dialing 1-877-344-7529 (home) or 1-412-317-0088 (worldwide) or Canada toll free at 1-855-669-9658. The replay convention ID quantity is 3788019.
About Aethlon and the Hemopurifier®
Aethlon Medical is a medical therapeutic firm centered on growing the Hemopurifier, a scientific stage immunotherapeutic gadget which is designed to fight most cancers and life-threatening viral infections and to be used in organ transplantation. In human research, the Hemopurifier has demonstrated the elimination of life-threatening viruses and in pre-clinical research, the Hemopurifier has demonstrated the elimination of dangerous exosomes from organic fluids, using its proprietary lectin-based know-how. This motion has potential functions in most cancers, the place exosomes might promote immune suppression and metastasis, and in life-threatening infectious ailments. The Hemopurifier is a U.S. Meals and Drug Administration (FDA) designated Breakthrough Machine indicated for the therapy of people with superior or metastatic most cancers who’re both unresponsive to or illiberal of normal of care remedy, and with most cancers varieties through which exosomes have been proven to take part within the growth or severity of the illness. The Hemopurifier additionally holds an FDA Breakthrough Machine designation and an open Investigational Machine Exemption (IDE) software associated to the therapy of life-threatening viruses that aren’t addressed with permitted therapies.
Further data will be discovered at www.AethlonMedical.com.
Ahead-Trying Statements
This press launch comprises forward-looking statements throughout the which means of Part 27A of the Securities Act of 1933 and Part 21E of the Securities Trade Act of 1934 that contain dangers and uncertainties. Statements containing phrases reminiscent of “might,” “imagine,” “anticipate,” “count on,” “intend,” “plan,” “challenge,” “will,” “projections,” “estimate,” “doubtlessly” or comparable expressions represent forward-looking statements. Such forward-looking statements are topic to important dangers and uncertainties and precise outcomes might differ materially from the outcomes anticipated within the forward-looking statements. These forward-looking statements are primarily based upon Aethlon’s present expectations and contain assumptions that will by no means materialize or might show to be incorrect. Elements that will contribute to such variations embrace, with out limitation, the Firm’s capability to boost further capital and to efficiently full growth of the Hemopurifier; the Firm’s capability to efficiently reveal the utility of the Hemopurifier in most cancers and infectious ailments and within the transplant setting; the Firm’s capability to attain and notice the anticipated advantages from potential milestones; the Firm’s capability to submit functions to and procure approval from the extra Ethics Committees in Australia and India, together with on the timing anticipated by the Firm; the Firm’s capability to provoke its deliberate oncology scientific trials in Australia and India, together with on the timing anticipated by the Firm; the Firm’s capability to handle and efficiently full its scientific trials, if initiated; the potential impression of Hemopurifier on the H5N1 Avian Influenza (H5N1 HPAI) virus in dairy cattle; the Firm’s capability to efficiently manufacture the Hemopurifier in ample portions for its scientific trials, and different potential dangers. The foregoing checklist of dangers and uncertainties is illustrative, however isn’t exhaustive. Further elements that would trigger outcomes to vary materially from these anticipated in forward-looking statements will be discovered below the caption “Threat Elements” within the Firm’s Annual Report on Kind 10-Ok for the 12 months ended March 31, 2024, and within the Firm’s different filings with the Securities and Trade Fee, together with its quarterly Stories on Kind 10-Q. All forward-looking statements contained on this press launch communicate solely as of the date on which they had been made. Besides as could also be required by legislation, the Firm doesn’t intend, nor does it undertake any responsibility, to replace this data to mirror future occasions or circumstances.
Firm Contact:
Jim Frakes
Interim Chief Govt Officer and Chief Monetary Officer
Aethlon Medical, Inc.
Jfrakes@aethlonmedical.com
Investor Contact:
Susan Noonan
S.A. Noonan Communications, LLC
susan@sanoonan.com
917-513-5303



SOURCE Aethlon Medical, Inc.
Disclaimer/Disclosure: Investorideas.com is a digital writer of third social gathering sourced information, articles and fairness analysis in addition to creates authentic content material, together with video, interviews and articles. Authentic content material created by investorideas is protected by copyright legal guidelines aside from syndication rights. Our website doesn’t make suggestions for purchases or sale of shares, companies or merchandise. Nothing on our websites ought to be construed as a suggestion or solicitation to purchase or promote merchandise or securities. All investing includes threat and doable losses. This website is at present compensated for information publication and distribution, social media and advertising, content material creation and extra. Disclosure: Athelon Medical, Inc. (AEMD) is a paid featured tech inventory on Investor concepts Extra disclosure: Contact administration and IR of every firm straight concerning particular questions.
Extra disclaimer information: https://www.investorideas.com/About/Disclaimer.asp Study extra about publishing your information launch and our different information companies on the Investorideas.com newswire https://www.investorideas.com/Information-Add/ and tickertagstocknews.com
World traders should adhere to rules of every nation. Please learn Investorideas.com privateness coverage: https://www.investorideas.com/About/Private_Policy.asp
Get extra biotech and medical tech information, articles, podcasts and inventory directories